(Within Science) — On July sixteen this yr, on what marks the 75th anniversary of the very first nuclear bomb examination, a affected individual may possibly go to the medical doctor for a coronary heart scan. A student may possibly open her textbook to research the intricate chemical pathways inexperienced vegetation use to switch carbon dioxide in the air into sugar. A curious grandmother may possibly spit into a vial for a genetic ancestry examination and an avid angler may possibly wake up to a wonderful early morning and make your mind up to fish at just one of his most loved lakes.
If any of these men and women were asked to feel about this choice of activities from their days, it would possible strike them as completely unrelated to the growing of a mushroom cloud over the New Mexico desert three-quarters of a century ago. But just about every product from the listing has been touched by that event.
The device that was detonated at dawn on that fateful day unleashed the vitality of around twenty,000 tons of TNT from a plutonium main roughly the measurement of a baseball. It obliterated the metal tower on which it stood, melted the sandy soil underneath into a greenish glass — and released the atomic age.
To access this milestone, the U.S. government had marshaled masses of men and women and put in billions in an work dubbed the Manhattan Challenge soon after the borough in New York wherever it was very first centered. Some of the influence of this wartime venture, these as the nuclear arms race, however looms significant in our community consciousness. But other impacts have pale from view for most of us.
The scan, the textbook, the genetic examination and the most loved lakeside retreat depict components of the Manhattan Project’s forgotten legacy. They are linked via a style of atom called an isotope, which was deployed in scientific labs and hospitals ahead of Earth War II, but whose overwhelming prevalence in the a long time soon after the war was enabled and pushed by the government apparatus that was a direct heir of the work to establish the bomb.
“Generally when equally standard men and women and scholars have thought about the legacy of the Manhattan Challenge, we thought about the way in which physics and engineering were set to military services use,” said Angela Creager, a science historian at Princeton College whose reserve “Life Atomic” chronicles the history of isotopes in the a long time soon after WWII. “Part of what I found was that atomic vitality had just as significantly of a legacy in some of the fields that we feel of as peaceable as it did in military services works by using. … A whole lot of the postwar improvements in biology and medication that have genuinely been taken for granted owe a whole lot to the supplies and insurance policies that were aspect of the Cold War U.S.”
Exact same Chemistry, Unique Physics
Isotopes were found in the early 20th century, all through a interval of amazing development in our understanding of subject. Scientists had confirmed the existence of the atom and were figuring out its three most important pieces — electrons, protons and neutrons — and how they match jointly. They sooner or later worked out that atoms could have the exact amount of protons, but a different amount of neutrons jammed jointly in their tiny nuclei.
These variants, called isotopes, are different flavors of the exact ingredient. Scientific shorthand picks letters to designate the ingredient — C for carbon, for illustration — and a amount to suggest the amount of protons as well as neutrons. C-fourteen for illustration, is applied for carbon with 6 protons and eight neutrons, when C-twelve is applied for the much more typical type of carbon that has 6 protons and 6 neutrons.
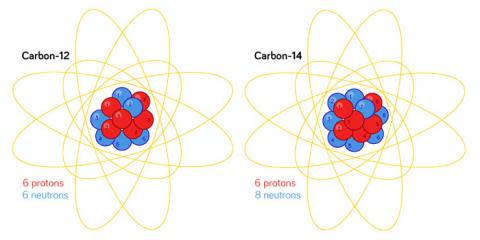
All carbon atoms have six protons in their nuclei. A radioactive carbon-fourteen atom has eight neutrons, when the much more typical carbon-twelve has only six. Whilst standard depictions of atoms demonstrate comparatively significant nuclei, they are, in actuality, tiny compared to the measurement of the electron cloud. (Credit history: Abigail Malate, Staff Illustrator)
Some isotopes are steady, existing for eons, when other individuals are unstable, or radioactive. These so-called radioisotopes sooner or later decay into some other ingredient or isotope, emitting radiation in the type of a particle or an energetic gamma ray in the procedure.
Prior to WWII, isotopes could be separated from pure substances, or they could be artificially produced by smashing accelerated charged particles from a device called a cyclotron into a target substance.
“What’s astounding about isotopes is that they are physically detectable, but chemically identical,” explained Creager. This meant scientists could switch an standard atom with an isotope cousin and then keep track of that atom via chemical or biological processes.
In the thirties, isotopes were deployed by scientists and medical professionals in a large variety of experiments, but their basic shortage retained the pool of users comparatively exclusive.
The frenetic thrust to establish an atomic bomb manufactured some thing that could generate significantly more substantial portions of isotopes — a nuclear reactor — and it improved the isotope landscape profoundly.

Exterior view of the nuclear reactor at Oak Ridge (Credit history: Department of Electricity via Flickr)
Uncle Sam’s Isotope Shop
In 1943, the U.S. military services constructed the first industrial-scale nuclear reactor in what grew to become the town of Oak Ridge, Tennessee. It served as a pilot plant for even more substantial reactors that were ultimately built in Hanford, Washington. The reactors’ most important job in the war work was to develop the isotope plutonium-239, which scientists had concluded could type the explosive main of just one of the two types of atomic bombs they were planning.
Plutonium-239 was a solution of the nuclear chain reactions propagating via the reactors’ gasoline slugs, modest cylinders of uranium encased in aluminum that were pushed into the experience of the reactor. Plutonium could be extracted by chemically processing the slugs soon after a selected volume of time in the reactor.
But plutonium was not the only isotope the reactors built other “by-product” isotopes were observed in the gasoline slugs. The scientists could also make bespoke isotopes by placing supplies into the reactor to be bombarded with flying neutrons that, like the charged particles in a cyclotron, could remodel the atoms they encountered. Most normally these reactor-built isotopes were radioactive.
Even all through the war, the Oak Ridge reactor in some cases produced isotopes for nonmilitary use, together with radioactive phosphorus-32, which was applied in cancer remedy.
Immediately after the war, quite a few Manhattan Challenge scientists argued that the Oak Ridge reactor should really start off consistently supplying isotopes to scientists and medical professionals for their exploration.
At the time, reactors had selected benefits over cyclotrons for this process: They could develop a larger quantity and diversity of isotopes. Furthermore, scientists were wanting to wrest handle of nuclear engineering from the military services by supplying it a peaceful application, Creager explained.
In 1946, an Isotope Department of the Manhattan Challenge was set up in Oak Ridge to oversee requests for isotopes. In June of that yr, an report in Science magazine marketed the availability of around 100 different isotopes.

Workers put together the very first sale of isotopes from the Oak Ridge reactor. The isotopes were shipped to the Barnard Free of charge Skin and Most cancers Hospital in St. Louis, Missouri. (Credit history: Department of Electricity via Flickr)
n a couple of many years, the Isotope Department (renamed the Isotope Division) was not only conference researchers’ need for isotopes, it was generating it. The Atomic Electricity Fee — the civilian successor to the Manhattan Challenge — marketed the application, lowered selling prices on some of the most broadly applied isotopes to underneath generation cost and offered coaching to scientists on how to correctly deal with radioactive supplies.
“I really don’t feel radioisotopes would at any time have been as broadly applied if it weren’t for the advertising of them,” Creager explained. “The [Oak Ridge] reactor had been constructed by the government as aspect of the Manhattan Challenge. Nobody who was acquiring radioisotopes was shelling out for that infrastructure. And even the generation and shipping charges were hugely subsidized by the government.”
By 1950, shipments of isotopes from Oak Ridge neared twenty,000. They significantly penetrated fields from medication to biochemistry.
Isotopes were the very first — and for much more than ten many years, the only — important civilian application of nuclear reactors, explained Nestor Herran, a science historian at Sorbonne College in Paris. Nuclear organizations normally touted them as illustrations of the beneficial and peaceful side of nuclear engineering and for a long time countrywide governments — together with the U.S.’s most important Manhattan Challenge collaborators Canada and the United Kingdom — were important players in the provide chain.
Alvin Weinberg, the director of Oak Ridge National Laboratory from 1955–1973, famously concluded: “If at some time a heavenly angel should really ask what the Laboratory in the hills of East Tennessee did to enlarge man’s everyday living and make it far better, I daresay the generation of radioisotopes for scientific exploration and clinical remedy will surely charge as a prospect for very first spot.”
A New Way of Seeing
On Aug. twelve, 1945, much less than a week soon after the bombings of Hiroshima and Nagasaki, the Dallas Morning Information ran a political cartoon displaying cancer, personified as a human skeleton, jogging from powerful rays of atomic vitality.
Whilst the cartoon may possibly seem naive to modern day eyes, it shows the optimism at the time that new nuclear technologies could be repurposed to give medical professionals powerful new resources to combat disorder. In 1948, the Atomic Electricity Fee released a application offering isotopes essentially for no cost for cancer prognosis, remedy and exploration. Recipients only had to fork out for shipping.
At very first, it was thought that radioactive isotopes may possibly attack cancer by concentrating in selected pieces of the overall body and irradiating tumors from the inside. In basic, this strategy did not perform as properly as hoped. In the 1950s, medical professionals turned much more to the isotopes cobalt-60 and cesium-137, which delivered exterior sources of radiation for cancer remedy. Other shorter-lived isotopes grew to become critical resources in diagnostic imaging to reveal patients’ interior anatomy. Nowadays, medical professionals around the environment perform around 40 million diagnostic procedures per yr with the most broadly applied clinical isotope: technetium-99.
Nuclear medication is possible isotopes’ most visible application to our peacetime lives, Herran explained.
When applied in diagnostic exams, isotopes demonstrate medical professionals concealed constructions inside the overall body. Far more typically, the energy of isotopes to reveal the unseen is most likely their most enduring legacy, and it was not just medical professionals who took advantage of this energy.
Isotopes can act as tiny atomic beacons that can be tracked via time when included to a system these as a mobile, a full organism or even an total planet’s environment.
The two steady and radioactive isotopes can serve as tracers, but at the end of WWII radioactive isotopes had some distinctive benefits for significant-scale use, and not only simply because governments had constructed resources that could develop them in significant portions.
Scientists could detect radioisotopes with comparatively simple gear like Geiger counters or X-ray film. “Had there only been steady isotopes obtainable, the degree of knowledge and the instrumentation necessary would have almost certainly limited isotope use to a scaled-down set of scientists,” Creager explained.
At the end of the war, isotopes — equally steady and radioactive — were the only instrument scientists had to keep track of particular person atoms and molecules via chemical transformations. Scientists significantly set them to use prying open some of nature’s black containers.
Just one black box at the time was photosynthesis — the procedure vegetation use to convert daylight, air and h2o into sugar. It was singled out as a problem ripe for unravelling with reactor-manufactured isotopes as early as 1944, in a report by Manhattan Challenge scientists on the potential postwar works by using of the government services.
In 1945, the chemist Melvin Calvin from the College of California, Berkeley commenced finding out photosynthesis employing the carbon-fourteen manufactured in the Berkeley cyclotron. Quickly soon after, he commenced getting C-fourteen from Oak Ridge. His team uncovered photosynthesizing inexperienced algae to C-fourteen tagged carbon dioxide. They then killed the algae soon after varying amounts of time and analyzed the chemical compounds that the vegetation had manufactured with the “hot” carbon. By 1958, Calvin and his colleagues, who until 1954 provided his most important collaborator Andrew Benson, had figured out just about every stage of the intricate chemical pathway, now termed the Calvin-Benson cycle, that most inexperienced vegetation use to convert carbon dioxide in the air into carbohydrates. In 1961, Calvin was awarded the Nobel Prize in chemistry for the perform.
Just one of the most important contributors to the scientists’ achievement was their use of paper chromatography to independent several compounds in the algae. When they held the paper up to clinical X-ray film, places shaped over the compounds that had included the radioactive carbon.
The physicist Freeman Dyson enthused about the procedure in a letter to household soon after he attended a converse by Calvin in 1948: “The very long-sighted men and women explained, when nuclear vitality very first arrived on the scene, that the application to biological exploration would be much more critical than the application to energy. But I doubt if any one predicted that items would actually get likely as speedy as they have.”
The Atomic Electricity Fee approximated that the Oak Ridge reactor could develop the exact amount of radioactive carbon-fourteen it would just take a thousand cyclotrons to make, and that the reactor could do it for about just one-10-thousandth the rate.
The Potential risks of Radioisotopes
In the nineteen forties, scientists largely thought about radiation risks in conditions of acute consequences, these as radiation burns or radiation poisoning from high degrees of publicity. It was only in the 1950s and ’60s that a much more comprehensive understanding of the potential for reduced-level radiation to lead to very long-phrase harm via genetic mutations emerged.

(Credit history: Shutterstock)
For that reason, in the early days of radioisotope tracer exploration, scientists were normally cavalier about the risks of radioactivity in the lab relative to later protection standards, Creager explained. Likewise, early clinical works by using of isotopes in some cases violated what would these days be thought of critical moral principles. A significantly unhappy illustration, Creager explained, is a research that gave radioiron to pregnant girls to keep track of how it was absorbed. The scientists applied radioiron from reactors, rather than cyclotrons, even even though the reactor-manufactured isotopes contained a extended-lived iron isotope that ultimately posed even bigger overall health risks. Even though the scientists who executed the authentic research did not feel that radioactive iron posed any threat to the fetuses, a later research observed that the women’s little ones had a modest but statistically important increase in the instances of childhood cancer. In actuality, this research contributed to an rising consciousness that fetuses are primarily prone to damage from radiation.
As expertise about the threats of radioisotopes amplified, so did regulation. Diagnostic works by using of radioisotopes these days typically expose clients to significantly lower amounts of radiation than in the early days and therapeutic works by using much more exactly target the tissue being taken care of.
Likewise, the advancement of far better radiation detection gear and the commercial generation of radio-labeled compounds also decreased the volume of radiation publicity scientists would usually get from conducting a radioisotope tracer experiment in the lab.
In general, when radioisotope tracers are taken care of appropriately, the degrees of radiation they generate are reduced compared to qualifications radiation. On the clinical side, quite a few medical professionals and clients conclude that the overall health gains they get from diagnostic nuclear medication outweigh the threats.
Still, it is critical to remain mindful about even reduced-dose exposures.
“You have to ask, ‘What threat is value getting, and who’s benefitting from the threat?’” Creager explained.
Existence From an Atom’s Stage of Watch
Calvin and Benson’s photosynthesis perform was at the vanguard of what grow to be a flood of exploration employing isotopes to uncover how everyday living features at the molecular level.
Even though C-fourteen experiments exposed a intellect-boggling multitude of metabolic pathways in residing organisms, radioactive phosphorus-32 allowed scientists to probe the properties of DNA. In 1952, Alfred Hershey and Martha Chase applied phosphorus-32 from Oak Ridge in aspect of their perform to demonstrate that DNA, rather than protein, is the things that helps make up genes. Whilst Hershey and Chase were not the very first to perform experiments that indicated DNA was the carrier of hereditary information and facts, theirs were the ones that certain most of the scientific group. Hershey was later a joint recipient of the 1969 Nobel Prize in Physiology or Medication.
In the following a long time, radioisotopes grew to become critical resources in genetics exploration, as scientists went on to find how DNA serves as a recipe for producing proteins, how people and animals share substantial swaths of genetic code, how selected diseases are linked to genetic mutations, and significantly much more.
“Radioisotopes had a huge influence in biology,” explained Allan Spradling, a developmental biologist at the Carnegie Institution for Science in Baltimore, Maryland. “They were just one of the important resources to go from an abstract view of genetics to real molecules and precise processes in the cells. It is really hard to feel of [a discovery] that would be centered just on radioisotope tactics, but a vast volume of our expertise of biology has at least a very good solid contribution from them.”
In addition to scientific studies of genetics and metabolic process, scientists also tracked nutrients and hormones via the human overall body, elucidating the roles of these vital atoms and molecules.
Isotopes’ energy to probe molecular processes didn’t cease at the boundaries of residing organisms both. In the field of radioecology, which grew substantially soon after WWII, scientists studied how atoms go via total ecosystems.
In the mid-nineteen forties, the ecologist G. Evelyn Hutchinson, an early adopter of isotope tactics, launched trace amounts of radioactive phosphorus-32 into the area waters of Linsley Pond in Connecticut to research how the nutrient cycled via the h2o, mud, algae and vegetation over the class of a couple of months. He started out the experiments with radioactive phosphorus from the Yale cyclotron, but ongoing later with shipments from Oak Ridge. The dependability and amount of the reactor-manufactured isotopes allowed him to get far better knowledge.
The experiments exposed how algae swiftly took up the included phosphorus. The algae grew and normally were swiftly eaten by tiny animals called zooplankton. When the algae and zooplankton died, they sank to the base of the pond, getting some of the phosphorus with them. Seasonal temperature alterations and winds could stir up the h2o column and carry the nutrient again to the area, completing the cycle. Just one implication of the findings was that far too significantly phosphorus may possibly toss the full system out of stability, leading to damaging blooms of harmful algae and photosynthesizing organisms called cyanobacteria.
Other ecologists studied how isotopes launched into the surroundings all through nuclear weapons generation and tests traveled via the surroundings. Just one significantly influential acquiring, centered largely on scientific studies near the Hanford reactors in Washington condition, exposed how radioactive contaminants could concentrate in animals and vegetation.
The ecologist Eugene Odum summarized the implications in his reserve “Fundamentals of Ecology”: “We could give ‘nature’ an evidently innocuous volume of radioactivity and have her give it again to us in a deadly offer.” This thought of bioaccumulation was later applied to other pollutants these as chemical pesticides.
“In my view, the use of radoisotopes made it achievable to evaluate quite a few varieties of costs of different ecological processes. P-32 and C-fourteen in particular were early breakthroughs. But steady isotopes have also built a significant influence on the scale of exploration that can be accomplished in the field,” explained Alan Covich, an ecologist at the College of Georgia in Athens.
Science historian Herran pointed out that ecologists’ use of isotopes in the 1950s helped cement a view of character as a sequence of networks that could be described by the movement of vitality and supplies.
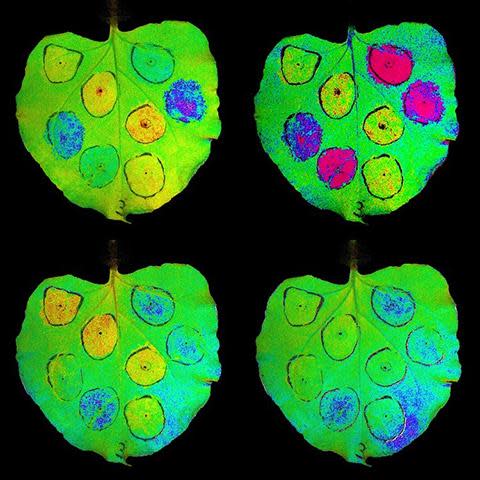
Tobacco leaves displaying transient overexpression of genes involved in a system that safeguards vegetation from light damage. By tweaking gene expression, plant biologists have bumped up crop efficiency. (Credit history: Lauriebeth Leonelli and Matthew Brooks/UC Berkeley)
The Legacy Carries on
The tale of how culture designs science — and how science designs culture — consists of plenty of twists. Shortly soon after Calvin claimed his eponymous cycle, an report in the Christian Century went so significantly as to forecast that the discovery would guide to “a vast increase in the world’s foodstuff provide within the following yr or so.”
It didn’t perform out that way. However, a short while ago, scientists have obtained some preliminary successes tweaking photosynthesis to increase crop yields, an work that has taken on rising importance as vegetation experience stresses from a warming environment, explained Krishna Niyogi, a plant biologist at the College of California, Berkeley. The scientists use resources, these as high-driven pc modeling and genetic engineering, that didn’t exist until current a long time.
Genetics exploration has exploded with applications to daily everyday living. Scientists and commentators in the mid-century never could have imagined today’s breadth of expertise in molecular biology, Spradling explained. Even though it is not possible to solitary out any just one procedure as liable for applications these as genetic tests, the full field owes a personal debt of gratitude to radioisotopes, he explained.
In other means, history, as they say, repeats alone. Knowledge about the way phosphorus in ponds and lakes can lead to algae blooms and fish die-offs helped guide to actions in the 1960s and ’70s to restrict phosphorus from sources these as laundry detergents, Covich explained. W.T. Edmondson, a former student of Hutchinson’s, campaigned to cleanse up Lake Washington, near Seattle, by diverting sewage, an additional significant source of phosphorus. The h2o good quality and overall health of the fish populations vastly improved. “But normally men and women fall again and neglect the basic science that concluded handle of all phosphorus sources is however necessary,” Covich explained. “Now we are acquiring much more harmful cyanobacteria as the phosphorus from sewage remedy vegetation and weather warming are generating an additional type of harmful brew.”
It is really hard to forecast how the scientific chain reactions touched by the Manhattan Project’s isotopes application will propagate via the following seventy five many years. But wanting again, quite a few scientists and historians concur that the consequences to date have been profound.
“I mainly arrived to the summary, in the class of researching my reserve, that radioisotopes would never have had the influence that they finished up possessing if not for Earth War II, which speedy-tracked the advancement of nuclear engineering on a huge scale,” Creager pointed out.
Radioisotopes extend the Manhattan Project’s legacy into realms of daily everyday living we really don’t normally hook up to the A-bomb. They demonstrate how physics and engineering link to biology and medication, how science back links to plan, and how an standard person’s standard working day back links to the pounds of human history. In that perception, these atomic tracers reveal not just how organic molecules remodel or how blood flows via a patient’s coronary heart they reveal the sinews of culture alone.
Acknowledgement: The creator would like to credit score Angela Creager’s reserve “Existence Atomic” for providing a wide overview of the topic, as properly as quite a few precise facts and penned offers pointed out in this tale.
Catherine Meyers is a deputy editor for Within Science. This tale originally appeared in Within Science. Click on below to go through the authentic tale.




